ELECTROCATALYSIS FOR ENERGY STORAGE AND CONVERSION
CO2 Electroreduction
 Within the last two decades CO2 sequestration and conversion has gained interest and is now essential in solving the global climate crisis. To date there have been several techniques for conversion of CO2 to commercial products, but CO2 electroreduction has become the most popular among them. Electrochemical reduction of CO2, an artificial way of carbon recycling, represents one promising solution for energy and environmental sustainability because of its ability to be driven by intermittent forms of energy (solar and wind), use of innocuous electrolytes and catalysts and its fuel cell having a compact and modular design. However, it is plagued with sluggish kinetics and high electrode potentials to generate high valued products. Additionally, more economical catalysts, like copper, lack selectivity for desired products. Our lab seeks to explore various catalytic systems that overcome these deficiencies and advances fundamental understanding in catalyst design and mechanism towards CO2 reduction.
Within the last two decades CO2 sequestration and conversion has gained interest and is now essential in solving the global climate crisis. To date there have been several techniques for conversion of CO2 to commercial products, but CO2 electroreduction has become the most popular among them. Electrochemical reduction of CO2, an artificial way of carbon recycling, represents one promising solution for energy and environmental sustainability because of its ability to be driven by intermittent forms of energy (solar and wind), use of innocuous electrolytes and catalysts and its fuel cell having a compact and modular design. However, it is plagued with sluggish kinetics and high electrode potentials to generate high valued products. Additionally, more economical catalysts, like copper, lack selectivity for desired products. Our lab seeks to explore various catalytic systems that overcome these deficiencies and advances fundamental understanding in catalyst design and mechanism towards CO2 reduction.
Fuel cell
We are developing a series of precious metal based catalyst by wet chemical synthesis for the cathode of fuel cell. Our aim is to find the champion structure and composition for ORR reaction, that is, high activity and high stability as well. We are now developing a new Co@Pt core-shell catalyst for ORR reaction, which shows 10 times higher activity than state-of-the-art Pt/C catalyst and the record stability (only 13% loss of mass activity after 30,000 cycles).
Metal-air batteries
We are looking for a sustainable and environmental friendly catalyst with high activity and long term stability for the cathode of metal-air batteries. By controlling the structure and composition, we are going to identifying the best catalyst for both ORR and OER, such as Pt-CoOx and Pd-NiFeOx hetero-structure catalyst.
HETEROGENEOUS CATALYSIS FOR SUSTAINABILITY
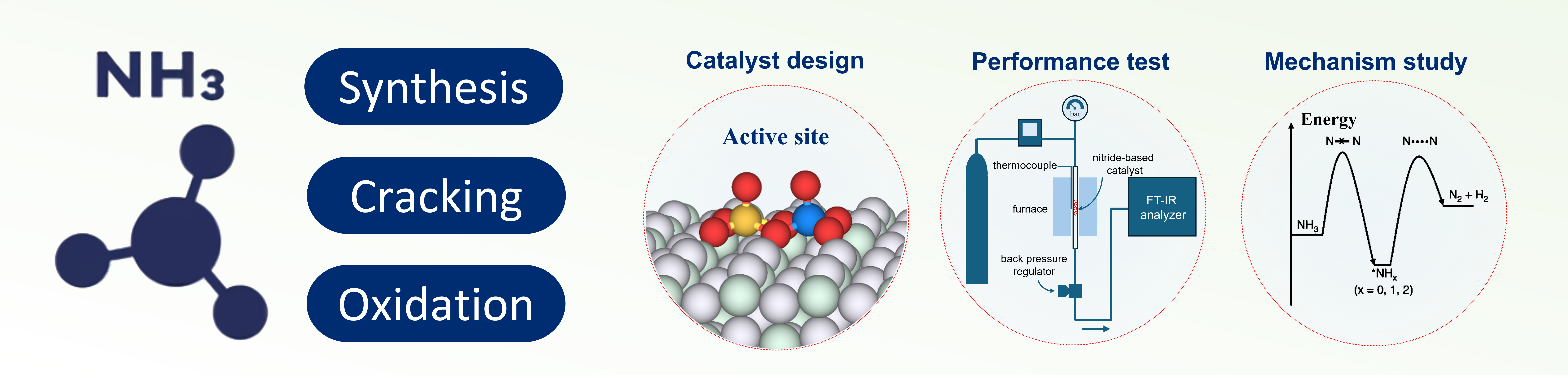
Design and Synthesis of Advanced Catalysts for Ammonia-Based Systems
Our research focuses on heterogeneous catalysis for energy and environmental applications, mainly involving the design, synthesis, and characterization of catalysts. Our goal is to develop advanced catalysts for energy systems using ammonia as an energy carrier, including ammonia synthesis, cracking, and oxidation, paving the way for the development and application of carbon-free energy systems.
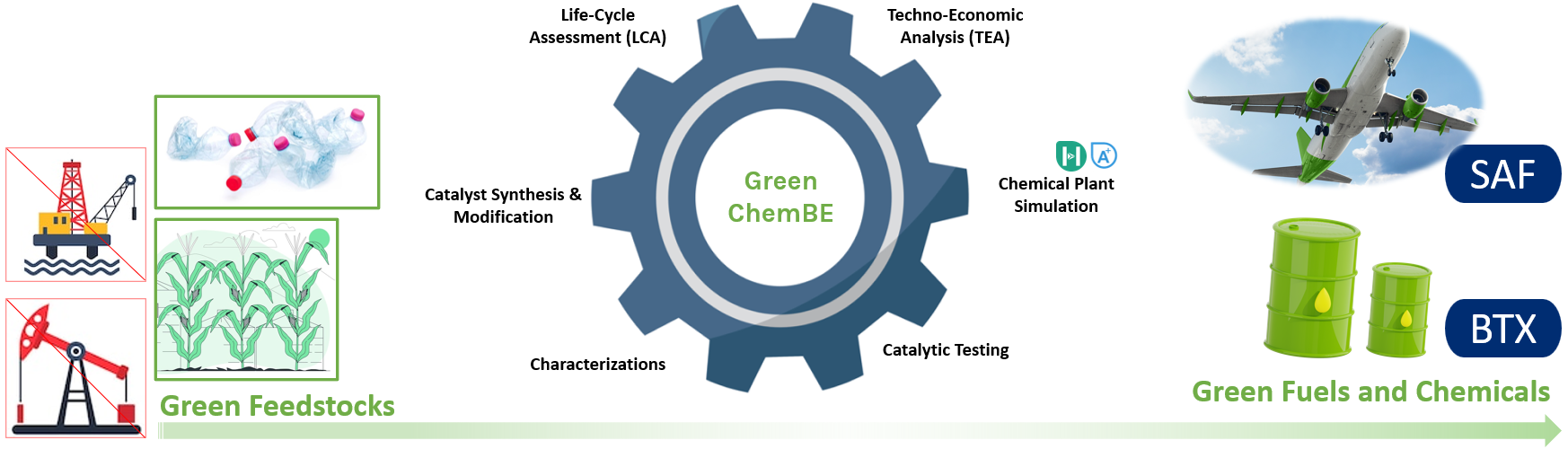
Green Chemical and Fuels Production
Our primary focus is to utilize green and sustainable feedstocks, such as plastic waste and biomass, to produce high-value chemicals and fuels with the aim of reducing carbon emissions. We have developed our own catalytic system, which includes the design, characterization, and testing of catalysts to assess their performance and understand the underlying mechanisms. Additionally, we thoroughly evaluate the scaling-up potential of the catalytic system by conducting techno-economic and life-cycle assessments using various chemical engineering software, such as ASPEN Plus, to ensure both environmental and economic benefits.
SYNTHESIS AND CHARACTERIZATION OF FUNCTIONAL NANOMATERIALS
We are developing advanced nanomaterials using organic or aqueous solution synthesis. Our aim is to control the size, shape, composition and heterostructures of these materials, by tuning the reaction conditions to tailor crystal nucleation and growth at the nanoscale. Our lab is also interested in microfluidics to advance the state of art in nanotechnology for different applications including sensing, diagnostic assays and drug delivery studies with the help of custom made microfluidic chips. Enabled by these new materials, we are seeking for functional applications in catalysis, energy storage and conversion, and many other fields, with particular interests in the following directions.